The United Kingdom’s state-run National Health Service (NHS) achieved an enormous milestone in the fight against cancer as it got ready to become the first healthcare system globally to offer a revolutionary cancer treatment injection that could potentially reduce treatment times by up to three-quarters.
The news came following the recent approval by the Medicines and Healthcare Products Regulatory Agency (MHRA) for the administration of an innovative cancer immunotherapy drug, atezolizumab, via a convenient “under the skin” injection method.
“This approval will not only allow us to deliver convenient and faster care for our patients but will enable our teams to treat more patients throughout the day,” stated Dr. Alexander Martin, a consultant oncologist at West Suffolk NHS Foundation Trust, as he expressed his excitement about this groundbreaking development.
Marius Scholtz, Medical Director at Roche Products Limited, emphasized the efficiency of the new injection method, explaining, “It takes approximately seven minutes, compared with 30 to 60 minutes for the current method of an intravenous infusion.”
It is expected to not only improve patient care but also increase the capacity for treating more patients in England.
How does this drug work?
Tecentriq, an antibody, works by binding to PD-L1, a molecule found on cancer cells and within tumors. PD-L1 normally interacts with PD-1 on T cells – the primary immune cells involved in killing cancer cells. This prevents excessive immune reactions. In cancer, this interaction stops T cells from attacking cancer cells. Tecentriq blocks PD-1/PD-L1 interaction, enabling active T cells to attack cancer cells.
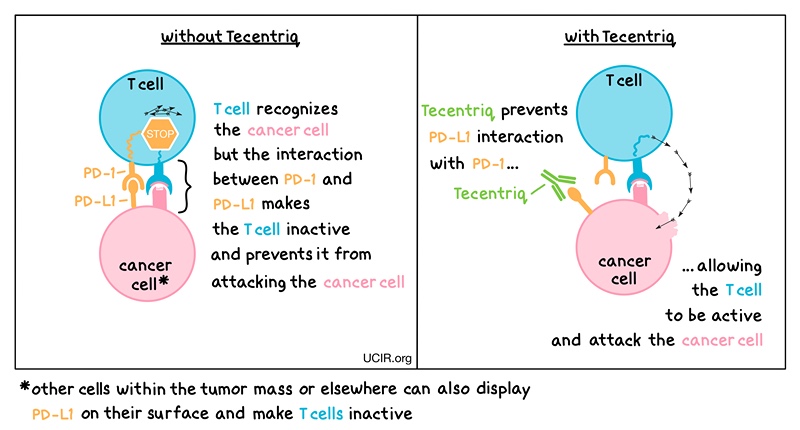
This game-changing medication is currently offered to NHS patients battling a variety of cancers, including lung, breast, liver, and bladder cancers, through intravenous infusion. With the adoption of the new injection method, NHS England anticipates that the majority of the approximately 3,600 patients starting atezolizumab treatment each year will transition to this time-saving approach.
Despite this exciting development, NHS England clarified that patients who are receiving intravenous chemotherapy in combination with atezolizumab may continue to receive their treatment through transfusion.
This historic decision by the NHS set a precedent for healthcare systems worldwide, demonstrating the potential for innovative approaches to revolutionize cancer care and save lives. As the world watches this pioneering achievement, it is evident that the United Kingdom remains at the forefront of healthcare innovation.